A05 Evolution of patient derived xenograft acute myeloid leukaemia subclones in a preclinical mouse model
Tumors consist of genetically and functionally heterogeneous cells and the most adverse subpopulation within a tumor determines the prognosis of cancer patients. While conventional anti-cancer treatment often substantially reduces tumor burden in the first weeks of treatment, a subpopulation of treatment-resistant cells might survive, persist over prolonged periods and ultimately induce relapse with dismal prognosis. Novel treatment options are urgently required to eliminate unfavourable leukemic clones.
We aim at characterizing the evolution of such adverse subpopulations in vivo in order to better understand which characteristics are associated with subclones of special challenge and which might determine patients' prognosis. Towards this aim, we will decipher the genetic, epigenetic and functional alterations of distinct subclones within samples derived from individual cancer patients. We will use acute myeloid leukaemias (AML) as model disease and genetic engineering in the individualized xenograft mouse model as cutting edge technical approach. Thereby we are able to study the behaviour and evolution of patients' cells growing in vivo in a clinic-close preclinical model. These studies finally allow developing novel therapeutic options against unfavourable subclones in AML.
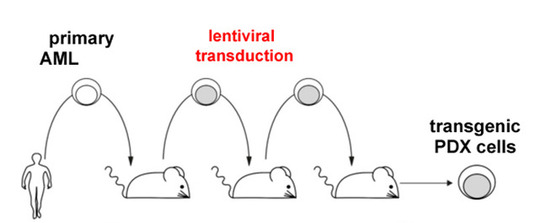
Figure legend: We transplant primary tumor cells from AML patients into severely immuno-compromised mice for amplification, and perform genetic engineering using lentivirusses.

Prof. Dr. Irmela JeremiasDr. von Haunersches Kinderspital, Ludwig-Maximilians-Universität München +49 (0)89 3187-1424 |

