A01 DNA modifications in the evolution of hematopoietic neoplasms
DNA modifications play a central role in the epigenetic regulation of gene expression in development and disease. While tumors at the time of diagnosis are well characterized, still very little is known about the evolution of neoplasms and in particular the role and dynamics of epigenetic changes before, during and after therapy.
We will investigate mechanisms of epigenetic deregulation focusing on the recently discovered new DNA modifications and will evaluate their contribution to the formation of hematopoietic neoplasms and the rise of therapy resistant tumor cells.
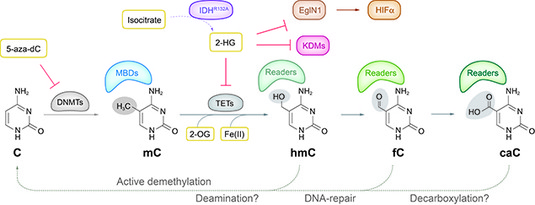
Figure legend: DNA (de-)modification pathways. 5-Methylcytosine (mC) was long considered a rather stable epigenetic mark. The recent discovery that TET proteins can oxidize mC to hmC, fC and caC indicated that DNA modifications are far more dynamic and complex as previously thought. There is now evidence for active demethylation through deamination, DNA repair and possibly also decarboxylation. These new DNA bases are recognized by several nuclear proteins that change during differentiation. Most interestingly, TET proteins depend on O2 and 2-oxogluterate (2-OG) from the citric acid cycle generated by IDH proteins providing a link to oxygen supply and metabolism. Mutations affecting DNMTs, TETs and/or IDHs were found in almost half of all AML samples. The frequently found IDH gain-of-function mutations lead to formation of 2-hydroxyglutarate (2-HG), which acts as competitive inhibitor of TET and other oxygenases, including KDMs and also e.g. EglNI that in turn affects HIF1alpha stability and hematopoiesis.

Prof. Dr. Heinrich LeonhardtFaculty of Biology, Ludwig-Maximilians-Universität München +49 (0)89 2180-74232 |

